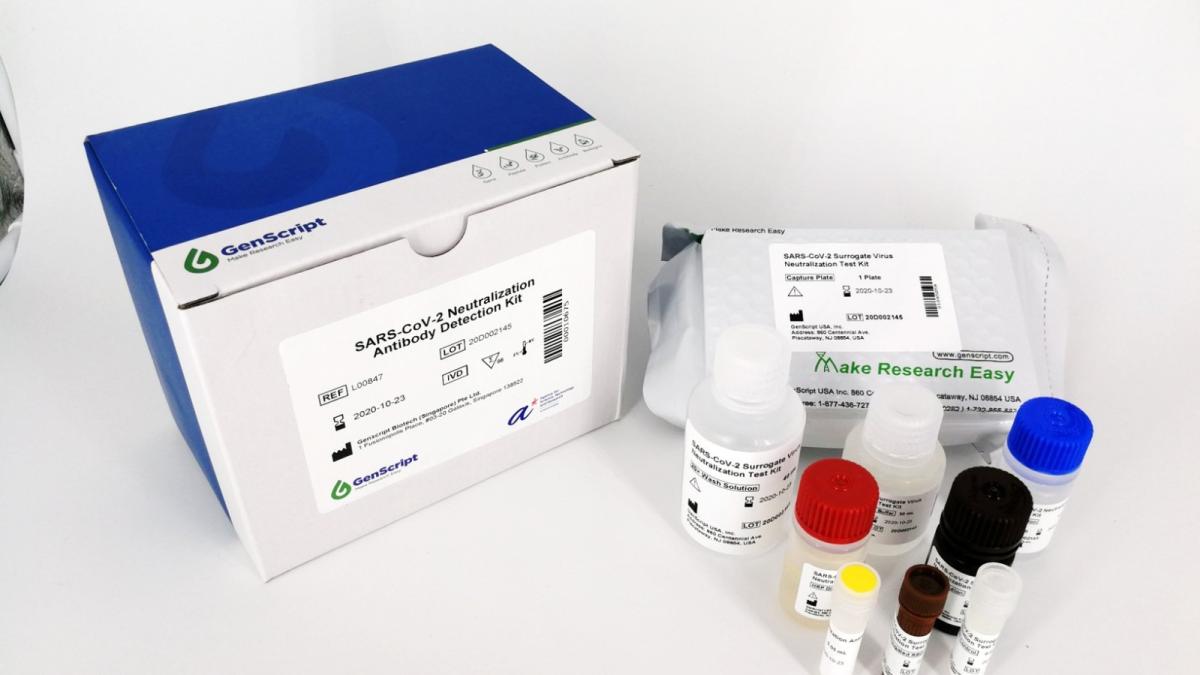
Biotech firm GenScript recognised for its pioneering neutralizing antibody test kits at SBR Management Excellence Awards
The company’s cPass™ test kits have equipped vaccine developers and residents in many nations, within and beyond Singapore, to fight the pandemic.
GenScript Biotech Corporation, the world's leading biology research service company, wins COVID Management Initiative of the Year at the SBR Management Excellence Awards in the Biotechnology category. The company has made significant contributions to the research and development of COVID-19 vaccines and test capabilities.
Presented by the Singapore Business Review, the Management Excellence Awards is honouring noteworthy individuals or teams who made a huge contribution to a business' success, as well as employee engagements and COVID management initiatives that made a positive impact on its workforce or customers.
GenScript specialises in gene synthesis technology, offering its products and services to partners and subsidiaries in the United States, China, Europe, Japan, South Korea, and Singapore. As a global biotechnology company, GenScript was steadfast in its commitment to supporting COVID-19 vaccine development. GenScript scaled up the production of genes, proteins, and peptides for vaccine developers around the world. In addition, GenScript launched the neutralization antibody test kit, cPass™ SARS-CoV-2 neutralization antibody detection kit, in partnership with Duke-NUS Medical School, and the Diagnostics Development Hub (DxD) at Singapore's Agency for Science, Technology and Research (A*STAR).
cPass™ detects whether someone has antibodies which neutralise the coronavirus, invented by local researchers, has become the first of its kind to receive Emergency Use Authorization (EUA) from the United States Food and Drug Administration (FDA).
cPass™ neutralization antibody test can estimate vaccine-induced protection for a population and also at an individual level. Triggering neutralising antibody response is a key success for vaccine developers. Unlike other serology tests, cPass™ detects neutralizing antibodies that are thought to prevent the coronavirus from infecting a patient's cells.
As the virus mutates, the need for boosters is imminent. cPass™ results can help clinicians formulate a risk based booster regimen for those who may need it urgently. Scientists also found that those aged 60 years and above may respond poorer to vaccination. Taking the cPass™ test two weeks and every three months after vaccination can then inform when the next booster may be needed.
The approval of cPass™ as the first commercial kit to determine neutralizing antibodies for Sars-CoV-2 by the FDA EUA is an incredible recognition for the Singapore research and biotech landscape. Since the launch of the kit, many clinics in Singapore have incorporated cPass™ test as part of the COVID-19 management and vaccine monitoring efforts. Residents in Singapore can visit their family doctor or specialist for a cPass™ test to understand their neutralizing antibody response to the vaccines or boosters.





















 Advertise
Advertise










